Abstract
Introduction: The survival of patients with chronic myeloid leukaemia (CML) treated with tyrosine kinase inhibitors (TKI) is now almost comparable to that of healthy population. Consequently, the financial and personal costs of life-long treatment, molecular monitoring and hospital visits have also increased. The ELN 2013 guidelines recommend molecular monitoring by BCR-ABL1\ABL1 quantitative reverse transcriptase polymerase chain reaction assay (RT-qPCR) every 3 to 6 months after the achievement of the major molecular response. We used our comprehensive institutional database of patients with CML to determine whether there is a group in whom the response is sufficiently deep and sustained so that they might reduce their frequency of hospital interactions.
Methods: We identified patients (pts) who had received TKI and reached a sustained MR4 (sMR4) and/or MR3 (sMR3) defined as a RT-qPCR <0.01% and <0.1% IS, respectively, maintained for at least one year. Patients who had received any chemotherapy (except from hydroxycarbamide), autologous or allogeneic transplants were excluded.
We defined loss of MR3 and MR4 as RT-qPCRs ≥0.1% and ≥0.01%, respectively, in two or more consecutive measurements. We calculated the median time to reach sMR3 and sMR4 from the date of starting TKI until the achievement of the first date of RT-qPCR <0.1% and <0.01% IS, respectively, maintained for at least 1 year. Cumulative incidence (CI) of loss of MR3 and MR4 after the first year of sustained response were calculated using the CI function. Patients were censored at last follow-up if on treatment or at the date of their first documented TKI interruption longer than 7 days.
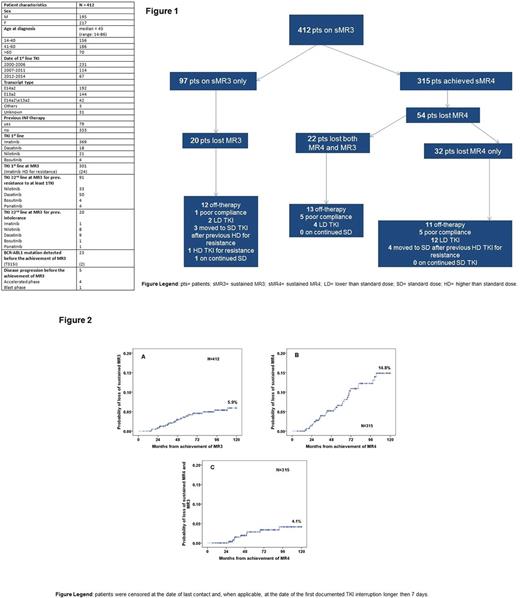
Results: 412 pts reached a sMR3 at any time of their TKI therapy (Table). Of these, 315 pts further improved their response to sMR4. The median time to achievement of sMR3 was 427 days (range 54-5533) and to sMR4 was 948 days (range 100-5164). Furthermore, pts who achieved sMR4 had achieved sMR3 more rapidly (median time 385 days vs 729 days, respectively). The median follow-up after the achievement of sMR3 was 1634 days (range 171-4391) for pts achieving MR3 only and 3115 days (range 199-5762) for those who reached sMR4. At last follow-up 42/412 pts had lost sMR3, 20 of whom had never achieved sMR4: only 1 of these 20 was on standard dose (SD) TKI. Of pts in sMR4, 54 lost sMR4 (including 22 who also lost sMR3). None of these 22 was on SD TKI at the time of loss of response. The TKI status at the time of loss of sMR3 or sMR4 is given in Figure 1. The CI of loss of sMR3 was 0.7%, 3.9% and 5.9% at 2, 5 and 10 years respectively (Figure 2A). The CI of loss of sMR4 was 1.4%, 6.6% and 14.8% at 2, 5 and 10 years respectively (Figure 2B). The CI of loss of sMR3 in those pts who had reached a sMR4 was 0%, 2.8% and 4.1% at 2, 5 and 10 years respectively (Figure 2C). After a median follow-up of 1172 days (range, 154-4422) from loss of sMR3, 3 of 42 pts had also lost CCyR. One of these lately progressed to blast crisis six years after starting first line Nilotinib and 1705 days after achieving sMR4, while on a reduced Nilotinib dose. One patient of the 97 who achieved only sMR3 but not sMR4 lost sMR3 whilst on SD TKI. None of the pts who lost sMR3 from sMR4 were on SD TKI at the time of loss. Although loss of sMR4 was seen in 4 pts on SD TKI, all 4 had previously received higher doses of TKI after evidence of resistance.
Conclusion: We chose loss of MR3 as our outcome as this is currently regarded as the measure of failure in trials of TKI discontinuation. We have shown that loss of MR3 remains unusual in pts with CML who have achieved deep and sustained responses, particularly in those who remain on standard dose TKI, who have not demonstrated prior resistance and who are known to be compliant. We are reluctant to suggest that sMR3 is a 'safe haven' for deep response, first because one such patient lost sMR3 on SD TKI and second because we have demonstrated that achievement of MR3 is slower in those destined to achieve only sMR3 and not sMR4, suggesting less responsive disease. We do however consider that sMR4 is a level of response that may justify less frequent monitoring in pts who are not considering a trial of treatment discontinuation.
If applied to clinical practise, our findings may have considerable impact on patient perception of disease and quality of life. Furthermore, it could provide financial savings.
Milojkovic: Novartis: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Incyte: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria. Foroni: Incyte: Honoraria, Research Funding; Ariad: Honoraria, Research Funding. Apperley: Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Therakos: Honoraria; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses , Research Funding, Speakers Bureau; Incyte: Honoraria; Sun Pharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal